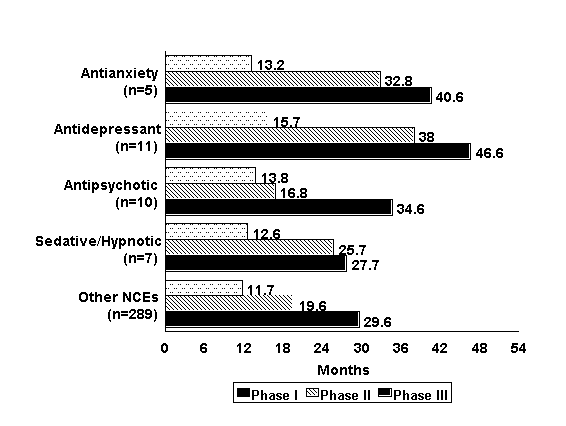
| Figure 1. |
 |
| Mean clinical phase development times for psychotropic and other new chemical entities (NCEs) approved in the United States during 1963 to 1997. The length of a phase is determined as the time from when the phase begins to when the next phase begins. Phase length for Phase III is the time from the start of Phase III to new drug application (NDA) submission. Only those NCEs with complete phase time data are included. |
published 2000