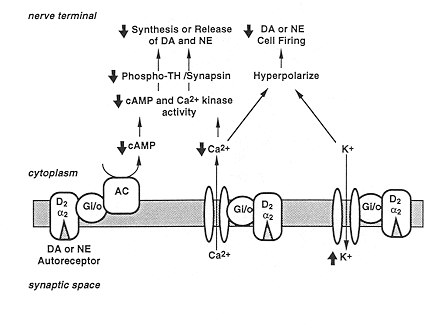
Schematic illustration of the mechanisms contributing to receptor regulation of neurotransmitter synthesis and release. The mechanisms which underlie the ability of catecholamine autoreceptors to inhibit neurotransmitter synthesis probably involve a combination of second messenger pathways and phosphoproteins. Illustrated in this figure are the effects of dopamine (DA) (D2) or norepinephrine (NE) (a2) autoreceptors on the cAMP and Ca2+ second messenger systems and ion channels. The activity tyrosine hydroxylase (TH), the rate limiting enzyme in the synthesis of both dopamine and norepinephrine, is increased by phosphorylation, which can occur in response to activation of a number of different kinases, including cAMP and Ca2+-dependent kinases (CaMK and PKC). Activation of D2 or a2 autoreceptors inhibits adenylyl cyclase and would be expected to reduce cAMP-dependent protein kinase activity and phosphorylation of TH. These autoreceptors may also inhibit Ca2+ channels and decrease the activity of Ca2+-dependent protein kinases and the level of phospho-TH.
One mechanism by which D2 and a2 autoreceptors may influence the release of neurotransmitters is by inhibition of Ca2+ channels. D2 or a2 autoreceptors may also hyperpolarize the nerve terminal by activation of K+ channels. Another mechanism by which these receptors may influence neurotransmitter release is by regulation of synaptic-vessicle associated proteins, such as synapsins. The synapsins are a family of proteins found in nerve terminals that are phosphorylated by cAMP and Ca2+/calmodulin-dependent protein kinases. Phosphorylation of synapsin results in a greater amount of neurotransmitter being released in response to a physiological stimulus. Decreased activity of cAMP or Ca2+/calmodulin-dependent kinases by activation of autoreceptors would be expected to decrease the phosphorylation state of synapsin and decrease the release of neurotransmitter.