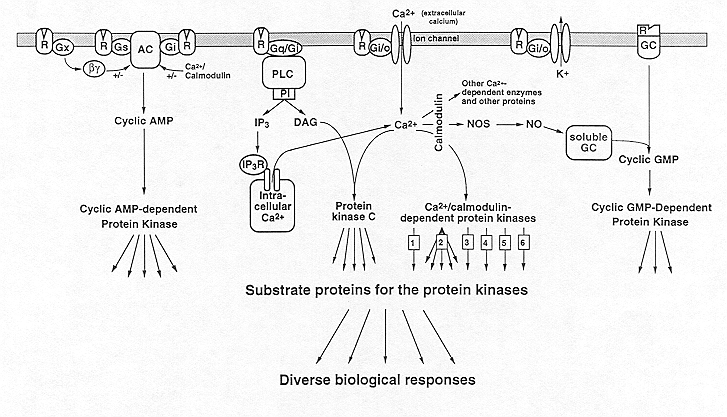
These second messengers, in turn, activate specific types of protein kinases. Brain contains one major type of cAMP-dependent protein kinase and of cGMP-dependent protein kinase, although subtypes of these enzymes are differentially expressed throughout the brain. These enzymes phosphorylate a specific array of substrate proteins, which can be considered third messengers. cAMP-dependent protein kinase has a broad substrate specificity, that is, it phosphorylates many substrate proteins and mediates most of the numerous second messenger actions of cAMP in the nervous system. The substrate specificity of cGMP-dependent protein kinase appears to be less broad, although by analogy with the cAMP system it is likely that it mediates many of the second messenger functions of cGMP. Brain contains two major classes of Ca2+-dependent protein kinase. One is activated by Ca2+ and calmodulin and is referred to as Ca2+/calmodulin-dependent protein kinase. Brain contains at least six distinct types of this enzyme: 1-4) Ca2+/calmodulin-dependent protein kinases I, II (several subtypes of this enzyme are known), III, and IV; 5) phosphorylase kinase; and 6) myosin light chain kinase. The other major class is activated by Ca2+ in conjunction with DAG and various phospholipids and is referred to as Ca2+/DAG-dependent protein kinase or protein kinase C; there are at least 7 closely-related variants of this enzyme present in the brain. Protein kinase C and Ca2+/calmodulin-dependent protein kinases II and perhaps I and IV have broad substrate specificities (as indicated by the multiple arrows in the figure) and each probably mediates many of the numerous second messenger actions of Ca2+ in the nervous system. [The figure also illustrates that some of the second messenger actions of Ca2+ in brain are mediated through proteins other than protein kinases.] Not shown in the Figure is the fact that some protein phosphatases are also subject to regulation by second messengers. For example, protein phosphatase 2B, or calcineurin, is activated upon binding Ca2+/calmodulin. Phosphorylation of substrate proteins by these various second messenger-dependent protein kinases alters their physiological activity in such a way as to lead to the biological responses of the extracellular messengers either directly or indirectly through intervening fourth, fifth, sixth, etc. messengers. Modified from Hyman and Nestler, 1993.